Membrane PTFE Filter Cartridges
3
LPTA filter cartridges are featured by permanently hydrophobic PTFE membrane, specially designed for sterile venting and gas application in vaccine manufacturing. They are integrity testable, offering the highest process security, high throughputs, extreme humidity and stringent in-line steam sterilizations. These filters can be steam-sterilizable repeatedly for long service life.
Features and Benefits
● High flow rates and low pressure drop
● High strength, long service life and cost-effective
● 100% integrity testable prior to release
Quality
● Cartridges produced in a controlled environment
● Manufactured according to ISO9001 certified Quality Management System
Specification
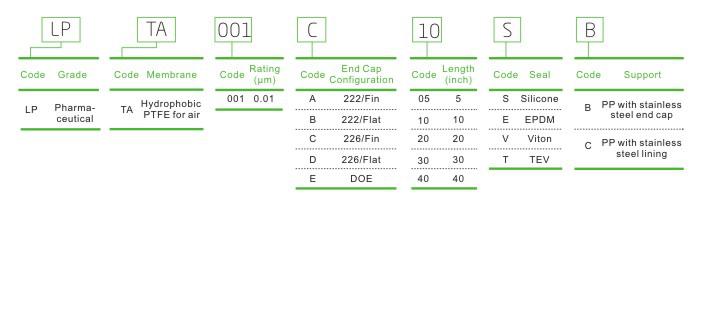
LPTA Filter Cartridge for Air/Gas in Vaccine Filtration
Share
LPTA filter cartridges are featured by permanently hydrophobic PTFE membrane, specially designed for sterile venting and gas application in vaccine manufacturing. They are integrity testable, offering the highest process security, high throughputs, extreme humidity and stringent in-line steam sterilizations. These filters can be steam-sterilizable repeatedly for long service life.
Features and Benefits
● High flow rates and low pressure drop
● High strength, long service life and cost-effective
● 100% integrity testable prior to release
Quality
● Cartridges produced in a controlled environment
● Manufactured according to ISO9001 certified Quality Management System
Specification
LPTA Filter Cartridge for Air/Gas in Vaccine Filtration
| Materials of Construction | |
| Membrane | Double-layer Polytetrafluoroethylene (PTFE) |
| Support | PP |
| Core, Cage and Drainage | PP |
| End Caps | PP (222/226 with encapsulated stainless steel reinforcing ring) |
| O-rings/Gasket | Silicone/EPDM/Viton/TEV |
| Sealing Technology | Thermal Bonding, No Adhesives |
| Dimensions | |
| Diameter | Φ68 |
| Length | 5 inch, 10 inch, 20 inch, 30 inch, 40 inch |
| Filtration Area, ft2 | |
| ≥ 5.8 per 10-inch element | |
| Pore Size, μm | |
| 0.01 (for gas) | |
| Maximum Differential Pressure | |
| Forward: 4.2 bar @ 23 ℃; 1.5 bar @ 85 ℃ | |
| Integrity Test- at 23 ℃ | |
| Bubble point | ≥ 1100 mbar with 60/40% IPA/water |
| HydroCorr (water intrusion test) | ≤ 0.75mL/min per 10-inch cartridge with water at 2620mbar |
| Bacterial Retention | |
| Passed the bacterial challenge testing using Brevundimonas diminuta(ATCC19146) at a minimum challenge concentration of 1 x 107 CFU/cm2. | |
| Extractables, per 10-inch cartridge | |
| ≤ 10 mg after 24-h soak in water | |
| Non-Fiber Releasing | |
| Meets the criteria for a “non-fiber releasing” filter as defined in 21 CFR 210.3 (b) (6). | |
| Toxicity | |
| Component materials meet GB/T 14233.2of Chinese National Standard for Safety Tests. | |
| TOC | |
| < 500 ppb after a water flush of 60 liters per 10-inch cartridge | |
| Multiple Sterilization Cycles | |
| 200 steam-in-place sterilization or autoclave cycles of 30 min at 123℃ | |
| Bacterial Endotoxins | |
| < 0.25 EU/ml as determined by the LAL test | |

TOP