Membrane PVDF Filter Cartridges
1
Share
Feature
LPSL filter cartridges are specially designed for sterilizing filtration of plasma fractionation. They are featuring extremely low extractables with no more than 10mg/10”, longer service life with 40 cycles of SIP. These filters can powerfully ensure final product quality.
Features and Benefits
● Double layer hydrophilic PES Membrane
● Low protein binding
● Extensive drug compatibility for critical applications
● Extremely low extractables
Quality
● Cartridges produced in a controlled environment
● Manufactured according to ISO9001 certified Quality Management System
Ordering Information
LPSL Low Extractable and Longer Service Life Filter Cartridge for Plasma Fractionation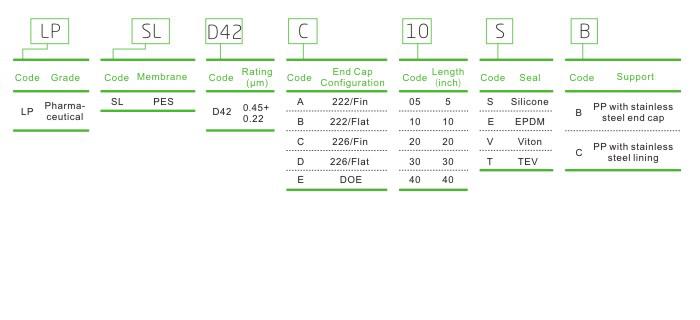
Application:
● Plasma Fractionation
● Sterile Injection Powder
● Vaccines
● Sterile Injection Powder
● Small & Large Volume Parenterals
● Plasma Fractionation
● Ophthalmic Solutions
● Facilities Water
● Cell Culture Media Preparation
● Active Pharmaceutical Ingredients
● Beer Filtration
● Wine Filtration
● Bottled Water Filtration
● Soft Drink Filtration
● Dairy Filtration
● High Fructose Syrup Filtration
● Semicon Filtration
● Ultrapure/DI Water Filtration
● Photoresist Filtration
● Display Filtration
● CMP Slurry Filtration
● Inkjet Inks Filtration
● Water Filtration
● Chemicals Filtration
Specification
LPSL filter cartridges are specially designed for sterilizing filtration of plasma fractionation. They are featuring extremely low extractables with no more than 10mg/10”, longer service life with 40 cycles of SIP. These filters can powerfully ensure final product quality.
Features and Benefits
● Double layer hydrophilic PES Membrane
● Low protein binding
● Extensive drug compatibility for critical applications
● Extremely low extractables
Quality
● Cartridges produced in a controlled environment
● Manufactured according to ISO9001 certified Quality Management System
Ordering Information
LPSL Low Extractable and Longer Service Life Filter Cartridge for Plasma Fractionation

Application:
● Plasma Fractionation
● Sterile Injection Powder
● Vaccines
● Sterile Injection Powder
● Small & Large Volume Parenterals
● Plasma Fractionation
● Ophthalmic Solutions
● Facilities Water
● Cell Culture Media Preparation
● Active Pharmaceutical Ingredients
● Beer Filtration
● Wine Filtration
● Bottled Water Filtration
● Soft Drink Filtration
● Dairy Filtration
● High Fructose Syrup Filtration
● Semicon Filtration
● Ultrapure/DI Water Filtration
● Photoresist Filtration
● Display Filtration
● CMP Slurry Filtration
● Inkjet Inks Filtration
● Water Filtration
● Chemicals Filtration
LPSL Low Extractable and Longer Service Life Filter Cartridge for Plasma Fractionation
| Materials of Construction | |
| Membrane | Double Layer Hydrophilic PES |
| Support | PP |
| Core, Cage and Drainage | PP |
| End Caps | PP (222/226 with encapsulated stainless steel reinforcing ring) |
| O-rings/Gasket | Silicone/EPDM/Viton |
| Sealing technology | Thermal Bonding, No Adhesives |
| Dimensions | |
| Diameter | Φ70 |
| Length | 5 inch, 10 inch, 20 inch, 30 inch |
| Filtration Area, ft2 | |
| ≥ 4.9 per 10-inch cartridge | |
| Pore Size, μm | |
| 0.45+0.22 | |
| Maximum Differential Pressure | |
| Forward: 4.2 bar @ 23 ℃; 1.5 bar @ 85 ℃ | |
| Integrity Test at 23 ℃ | |
| Water bubble point | ≥ 3500 mbar (50 psig) |
| Diffusive flow | ≤ 30ml/min/10”@2.8bar |
| Bacterial Retention | |
| Passed the bacterial challenge testing using Brevundimonas diminuta (ATCC19146) at a minimum challenge concentration of 1 x 107 CFU/cm2. | |
| Extractables, per 10-inch cartridge | |
| ≤ 10 mg after 24-h soak in water | |
| Effluent Particle Level | |
| Meets the requirements of Chinese Pharmacopoeia 2010, Volume Ⅱ, appendix Ⅸ C. | |
| Non-Fiber Releasing | |
| Meets the criteria for a “non-fiber releasing” filter as defined in 21 CFR 210.3 (b) (6). | |
| Toxicity | |
| Component materials meet GB/T 14233.2of Chinese National Standard for Safety Tests. | |
| TOC | |
| < 500 ppb after a water flush of 60 liters per 10-inch cartridge | |
| Multiple Sterilization Cycles | |
| 40 steam-in-place sterilization or autoclave cycles of 30 min at 123℃ | |
| Oxidizable Substances | |
| Meets the criteria of Chinese Pharmacopoeia 2010, volume Ⅱ for purified water. | |
| Bacterial Endotoxins | |
| < 0.25 EU/ml as determined by the LAL test | |
TOP