Membrane MCE Filter Cartridges
2
Share
Feature
LPM filter cartridges are characterized by high-performance, hydrophilic mixed cellulose esters membrane, offering high flow rates, low protein adsorption and superior microbiological safety. These filters are designed to reduce costs and are especially suited for in prefiltration of vaccines.
Features and Benefits
● Low protein binding
● Superior throughputs and cost-effective
● High retention efficiency for more critical degrees of prefiltration
Quality
● Cartridges produced in a controlled environment
● Manufactured according to ISO9001 certified Quality Management System
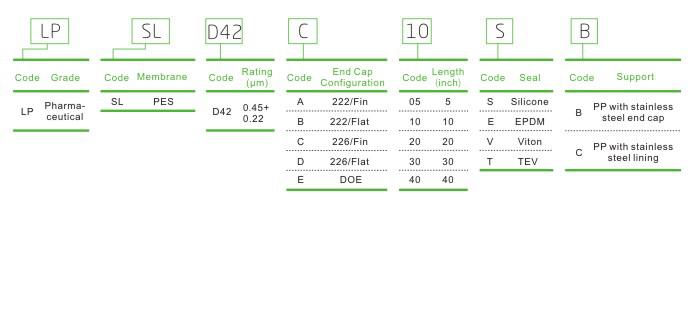
Ordering Information
LPSL Low Extractable and Longer Service Life Filter Cartridge for Plasma Fractionation
Application:
• Plasma Fractionation
• Sterile Injection Powder
• Vaccines
• Sterile Injection Powder
• Small & Large Volume Parenterals
• Plasma Fractionation
• Ophthalmic Solutions
• Facilities Water
• Cell Culture Media Preparation
• Active Pharmaceutical Ingredients
• Beer Filtration
• Wine Filtration
• Bottled Water Filtration
• Soft Drink Filtration
• Dairy Filtration
• High Fructose Syrup Filtration
• Semicon Filtration
• Ultrapure/DI Water Filtration
• Photoresist Filtration
• Display Filtration
• CMP Slurry Filtration
• Inkjet Inks Filtration
• Water Filtration
• Chemicals Filtration
Specification
LPM filter cartridges are characterized by high-performance, hydrophilic mixed cellulose esters membrane, offering high flow rates, low protein adsorption and superior microbiological safety. These filters are designed to reduce costs and are especially suited for in prefiltration of vaccines.
Features and Benefits
● Low protein binding
● Superior throughputs and cost-effective
● High retention efficiency for more critical degrees of prefiltration
Quality
● Cartridges produced in a controlled environment
● Manufactured according to ISO9001 certified Quality Management System
Ordering Information
LPSL Low Extractable and Longer Service Life Filter Cartridge for Plasma Fractionation

Application:
• Plasma Fractionation
• Sterile Injection Powder
• Vaccines
• Sterile Injection Powder
• Small & Large Volume Parenterals
• Plasma Fractionation
• Ophthalmic Solutions
• Facilities Water
• Cell Culture Media Preparation
• Active Pharmaceutical Ingredients
• Beer Filtration
• Wine Filtration
• Bottled Water Filtration
• Soft Drink Filtration
• Dairy Filtration
• High Fructose Syrup Filtration
• Semicon Filtration
• Ultrapure/DI Water Filtration
• Photoresist Filtration
• Display Filtration
• CMP Slurry Filtration
• Inkjet Inks Filtration
• Water Filtration
• Chemicals Filtration
LPSL Low Extractable and Longer Service Life Filter Cartridge for Plasma Fractionation
| Materials of Construction | |
| Membrane | Hydrophilic MCE |
| Support | PP |
| Core, Cage and Drainage | PP |
| End Caps | PP (222/226 with encapsulated stainless steel reinforcing ring) |
| O-rings/Gasket | Silicone/EPDM/Viton |
| Sealing Technology | Thermal Bonding, No Adhesives |
| Dimensions | |
| Diameter | Φ68 |
| Length | 5 inch, 10 inch, 20 inch, 30 inch, 40 inch |
| Filtration Area, ft2 | |
| ≥ 6.4 per 10-inch element | |
| Pore Size, μm | |
| 0.1, 0.22, 0.45, 0.65, 1.0, 3.0, 5.0 | |
| Maximum Differential Pressure | |
| Forward: 4.2 bar @ 23 ℃; 1.5 bar @ 85 ℃ | |
| Bacterial Retention (for 0.1 and 0.22 μm only) | |
| Passed the bacterial challenge testing using Brevundimonas diminuta(ATCC19146) at a minimum challenge concentration of 1 x 105 CFU/cm2. | |
| Extractables, per 10-inch cartridge | |
| ≤ 50 mg after 24-h soak in water | |
| Effluent Particle Level | |
| Meets the requirements of Chinese Pharmacopoeia 2010, Volume Ⅱ, appendix Ⅸ C. | |
| Non-Fiber Releasing | |
| Meets the criteria for a “non-fiber releasing” filter as defined in 21 CFR 210.3 (b) (6). | |
| Toxicity | |
| Component materials meet GB/T 14233.2of Chinese National Standard for Safety Tests. | |
| TOC | |
| < 500 ppb after a water flush of 60 liters per 10-inch cartridge | |
| Multiple Sterilization Cycles | |
| 15 steam-in-place sterilization or autoclave cycles of 30 min at 123℃ | |
| Oxidizable Substances | |
| Meets the criteria of Chinese Pharmacopoeia 2010, volume Ⅱ for purified water. | |
| Bacterial Endotoxins | |
| < 0.25 EU/ml as determined by the LAL test | |
TOP